|
Daniel Barrera
Contact: |
I am a first year Plant Systems Biology IGERT student. I graduated from the University of Kansas in 2008 with a B.S. in Biochemistry. I was a member of the Ronald E. McNair research scholars program at my school where I wrote proposals, conducted and presented research for an organic chemistry lab. When I'm not working, my spare time is spent with my friends and loved ones. I like exercise- soccer and basketball are my favorite sports to play. Any appreciable spare time is devoted to traveling. I love visiting our national parks, camping, and enjoying nature. I don't think I could go a winter season without taking a ski lift.
Previous Research:
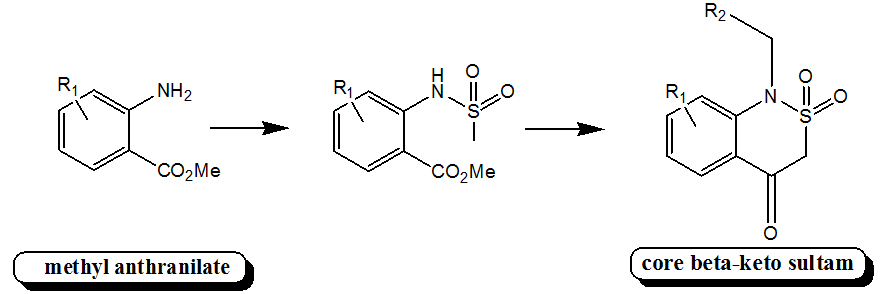
I worked for Dr. Paul Hanson at KU where I synthesized novel sultam scaffolds and subsequently developed combinatorial libraries of these compounds. The purpose of the work was to develop facile routes toward large numbers of structurally similar but distinct drug-like compounds for biological screening assays. The generation of the core scaffold involved utilizing low-cost starting materials (methyl anthranilate) and high-yield coupling reactions. This afforded us a stable sultam with numerous reactive 'handles' for simple yet diverse modifications.
Methyl anthranilate by itself is a commercially available compound that consists of an aromatic amine with a methyl ester group positioned ortho to the amino group. Or, more simply, it is the methyl ester of anthranilic acid. Sulfonylation of this compound using mesyl chloride yields a sulfonamide. At this point, the compound can be alkylated at the amino group, and then cyclized via the Dieckman condensation reaction. The product of this reaction is the core sultam.