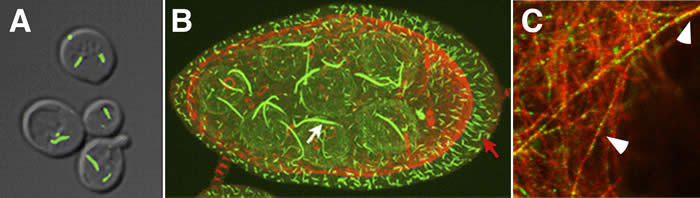
Systems biology approaches to identifying novel intracellular structures
The discovery of large supra-molecular complexes, such as the purinosome, has suggested that this form of subcellular organization is central to enzyme regulation. In order to address this question, we screened the yeast GFP strain collection to identify proteins that can assemble into visible structures. This screen identified a large number of intracellular structures including four novel filament systems comprised of glutamate synthase, GDP-mannose pyrophosphorylase, CTP synthase, or subunits of the eIF2/2B translation factor complex. The recruitment of CTP synthase to filaments and foci can be modulated by mutations and regulatory ligands that alter enzyme activity arguing that the assembly of these structures is related to control of CTP synthase activity. CTP synthase filaments are evolutionarily conserved and are restricted to axons in neurons. This spatial regulation suggests that these filaments have additional functions separate from the regulation of enzyme activity. The identification of four novel filaments greatly expands the number of known intracellular filament networks and has broad implications for our understanding of how cells organize biochemical activities in the cytoplasm.
Polymerization as a novel mode of enzyme regulation.
In our initial studies of intracellular filaments made up of Ura7p/CTP synthase, we found that one of the triggers for polymerization is end-product inhibition of the enzyme. This suggested that polymerization is intimately connected to regulation of enzymatic activity. However, this work also raised the question of why self-assembly of an inhibited enzyme into a polymer would be advantageous for promoting enzyme regulation. We propose that this form of enzyme regulation functions analogously to the way in which microtubules and actin operate – the core of the polymer is locked into a particular conformation by end limited disassembly. In conventional end-product inhibition for Ura7p/CTP synthase, the equilibrium binding to CTP is the sole determinant of the degree of inhibition. However, since inhibited CTP synthase is capable of polymerization, the enzyme must not only release CTP, it must also exit the polymer – a process that is dependent on the number of filament ends. As a result inhibited enzyme can be “locked” into an inactive state by the act of polymerization generating “switch-like” regulation of the enzyme. Since we have identified a number of enzymes that exhibit polymerization in vivo, it is likely that this mode of regulation is not unique to Ura7p/CTP synthase. We are currently using live imaging in concert with mutant analysis to test key aspects of this model for Ura7p/CTP synthase.