Research
NEWS: We are in the process of updating our website. We are working on exciting new projects about the maize cuticle and maize pathogen response. Stay tuned!
Laurie Smith’s lab investigates cellular mechanisms governing the orientation of plant cell division and growth, mainly in the context of maize leaf development. Current projects are outlined below.
Asymmetric Cell Division
Asymmetric cell divisions are those that produce daughters of different sizes, shapes and/or developmental fates (see illustration below). In plants, as in other eukaryotes, asymmetric divisions are associated with pattern formation during embryogenesis, establishment of new cell lineages, formation of specialized cell types, and maintenance of stem cell populations. In all of these processes, developmental asymmetry is closely tied to division polarity, which is oriented by either intrinsic or extrinsic cues. Most work on asymmetric cell division in plants has focused on how the divergent fates of daughter cells are specified. In contrast, we are interested primarily in how the physical asymmetry of the division is achieved. For example, what are the polarizing cues? How are they perceived? How is this perception translated into premitotic polarization of the mother cell? How is the division plane oriented in relation to mother cell polarity?
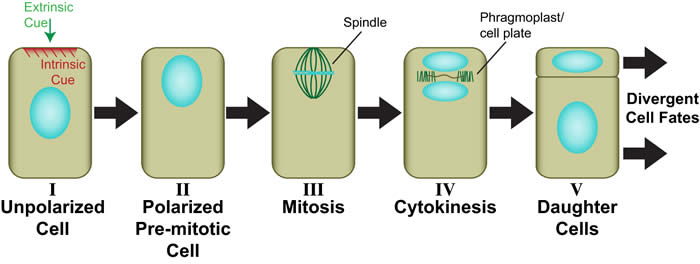
Generic view of plant asymmetric cell division (Facette and Smith, 2012). Perception of an intrinsic or extrinsic polarizing cue (I) leads to polarization of the mother cell including migration of the premitotic nucleus into the future division plane (II). The dividing nucleus is retained in the asymmetric division plane (III), where a new cell wall (cell plate) ultimately forms during cytokinesis through the action of the phragmoplast (IV). Daughter cells with different sizes and/or shapes are formed (V), which will adopt different developmental fates.
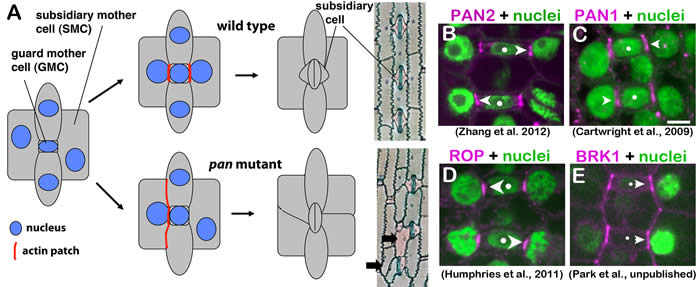
Features of stomatal development in maize. (A) Stomatal formation involves two classes of asymmetric division: one that forms a guard mother cell (GMC) and another that forms adjacent subsidiary cells (described in more detail below). Perturbation of premitotic subsidiary mother cell (SMC) polarization in pan1 and pan2 mutants (also in rop and brk mutants, not illustrated) leads to aberrant subsidiary cell formation. PAN2 (B), PAN1 (C), Type I ROP GTPases (D) and the putative SCAR complex subunit BRK1 (E) all localize asymmetrically within premitotic SMCs. Dots mark GMCs and arrowheads point to sites of localized accumulation of each protein within adjacent SMCs at sites of GMC contact.
Stomatal development in maize provides an excellent system for addressing these questions. Stomatal complexes in grasses such as maize consist of pair of guard cells, which are flanked by a pair of subsidiary cells that participate in regulation of stomatal opening. These stomata form through an invariant sequence of coordinated asymmetric cell divisions (see illustration above for wild type in A). The guard mother cell (GMC, itself the product of an asymmetric division) induces and polarizes divisions of neighboring subsidiary mother cells (SMCs) toward the GMC. SMC polarization is marked by the formation of dense patches of cortical F-actin at the GMC contact site and nuclear migration to that site. After mitosis, asymmetric cytokinesis in SMCs forms the subsidiary cells, and then the GMC divides longitudinally to form a guard cell pair. Analysis of mutants has demonstrated that two leucine-rich repeat receptor-like kinases (LRR-RLKs), PAN1 and PAN2, promote SMC polarization. Both proteins are themselves localized asymmetrically within SMCs at sites of GMC contact (see B and C, above; Cartwright et al., 2009; Zhang et al., 2012). PAN2 appears to act upstream of PAN1 since PAN1 localization at GMC contact sites requires PAN2 but not vice versa, however we found no evidene of a physical interaction between PAN1 and PAN2. These receptor-like proteins may be involved in perception of polarizing cues from adjacent GMCs, or may function somehow to amplify or stabilize SMC polarity downstream of the initial perception of GMC-derived polarizing cues. However, PAN1 and PAN2 ligands are unknown.
Small Rho-family GTPases (ROPs) were previously known to play key roles in polarization of plant cell growth. In collaboration with John Fowler at Oregon State University, we recently showed that PAN1 functions with Type I ROPs to promote the premitotic polarization of SMCs (Humphries et al., 2011). As illustrated in D, above, ROPs localize within SMCs at GMC contact sites. This localization occurs after PAN1 and PAN2 and requires both LRR-RLKs. PAN1 physically interacts with ROPs though the interaction may be indirect. Our work demonstrated for the first time a role for ROP GTPases in polarization of cell division, and provided new insight into mechanisms of ROP recruitment/activation at specific intracellular sites. A schematic model summarizing molecular features of SMC polarization is shown below.
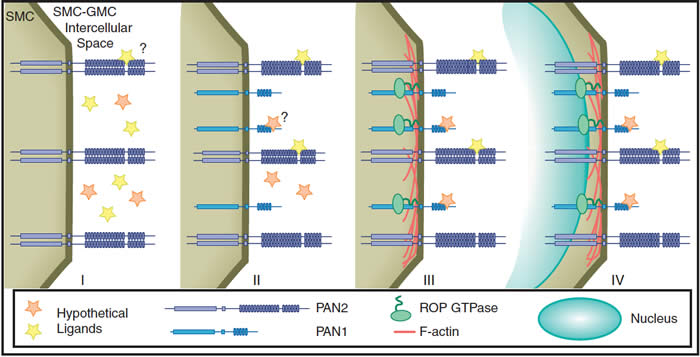
Molecular events in SMC polarization (Facette and Smith, 2012). Initially, GMCs produce an extrinsic cue, possibly one or more ligands represented by stars (panel I). The GMC-derived cue is perceived by the SMC, possibly via binding of a ligand to locally accumulated PAN2. Next, PAN1 accumulation occurs. PAN1 may perceive the same signal as PAN2, or a different ligand, or may not directly interact with any ligand (panel II). Subsequently, ROP GTPases and F-actin accumulate at the SMC–GMC contact site via interaction with PAN1 (panel III). Finally, the SMC nucleus migrates to the SMC–GMC contact site (panel IV).
A current project is investigating the role of the SCAR complex in this pathway. Mutants lacking BRK1/HSPC300 or BRK3/NAP1 subunits of a putative SCAR complex, a positive regulator of the actin-nucleating ARP2/3 complex, have similar SMC polarization defects as pan mutants. The mammalian SCAR complex is regulated by Rho-family GTPases, and plant SCAR complexes have been shown to interact with ROPs. Thus, we hypothesized that SCAR might act downstream of ROPs at GMC contact sites of SMCs, mediating ROP-dependent F-actin accumulation there to promote SMC polarization. Consistent with that hypothesis, BRK1 localizes to GMC contact sites of SMCs (see E in middle figure above). Surprisingly, however, further investigation of this hypothesis indicates that the SCAR complex acts upstream rather than downstream of PANs and ROPs. Thus, SCAR (and presumably ARP2/3 complex)-dependent actin nucleation is implicated in the mechanism by which PAN1 and PAN2 become asymmetrically localized in SMCs, while downstream events linking ROPs to actin nucleation and SMC polarization remain as yet unknown.
Future work will focus on understanding PAN1 and PAN2 polarization and the role of actin in this process. We are also working on identifying and analyzing additional components of the pathway linking hypothetical GMC-derived polarizing cues to SMC polarization and asymmetric division. Current approaches emphasize proteomic strategies (e.g. identification of proteins whose phosphorylation depends on PAN1 and/or PAN2, and identification of proteins that co-IP with PAN1 and/or PAN2), complemented by genetics, biochemistry and cell biology.

Maize Proteome Analysis
In collaboration with Steven Briggs and Vineet Bafna’s groups at UCSD, we are participating in an NSF Plant Genome Program project with the following aims and results to date:
Aim 1: Create an Atlas of Maize Proteins. The atlas will include the identity and relative amount of tens of thousands of proteins and phosphoproteins in >30 different tissues and stages of maize development. The atlas will also include the protein composition of the plasma membrane, chloroplast, mitochondrion, and peroxisome.
Data on the distribution and quantification of >13,000 proteins and 4,000 phosphorylated proteins from 8 tissues representing different stages and compartments of developing maize seeds is available in a searchable form at maizeproteome.ucsd.edu. An analysis of these data was recently submitted for publication (J. Walley et al.). Collection and analysis of data from many plant tissues and subcellular fractions is underway. The Smith lab has focused on analysis of the developing leaf proteome (consisting of >12,000 proteins and 3,000 phosphorylated proteins, with findings summarized as follows (abstract from M. Facette et al., 2013):
We carried out large-scale, quantitative analyses of maize leaf proteotypes at four developmental stages. Exploiting the developmental gradient of maize leaves, we analyzed protein and phosphoprotein abundance as maize leaves transition from proliferative division to differentiation to cell expansion, and compared these developing zones to one another and the mature leaf blade. A small portion of the proteomes differ between growing zones, but comparison of phosphoproteomes reveals many proteins uniquely phosphorylated in a single zone, suggesting a key role for post-translational regulation in developmental transitions. Analysis of proteins with cell wall- and hormone-related functions illustrate the utility of the dataset and provide further insight into aspects of maize leaf development. We highlight novel phosphorylation sites within these proteins, many of which are conserved in Arabidopsis. We also discuss instances where comparison of phosphosphorylated and unmodified peptides from a particular protein indicates tissue-specific phosphorylation. For example, we observe tissue-specific changes in the phosphorylation status of ZmPIN1. These changes correlate with changes in PIN1 polarization in epidermal cells during development, suggesting a unique role for the corresponding phosphosite in PIN1 regulation. Together, our data provide new insights into regulatory processes underlying maize leaf development, and provide a community resource cataloging the abundance and phosphorylation status of thousands of maize proteins at four leaf developmental stages.
Aim 2: Use peptide data to improve maize genome annotation via discovery, revision, and/or confirmation of maize gene models. Analysis of peptide data from seed tissues has so far led to 165 previously unidentified protein-coding genes and revision of 742 gene models (N. Castellana et al., in preparation).