Unpublished lab protocol 12/1993 to 3/1997
Kazuyuki Kuchitsu, John M. Ward, Mark Ellisman*, Ilona Schelle
and Julian Schroeder
University of California, San Diego
Department of Biology and Center for Molecular Genetics
*UCSD National Center for Microscopy and Imaging Research
La Jolla, CA 92093-0116, USA
correspondence: Julian
Schroeder
(julian@biomail.ucsd.edu)

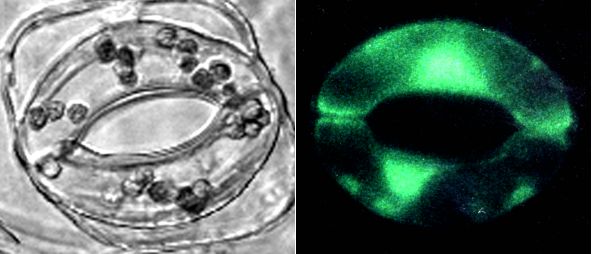
Figure 1: left: bright field image of Commelina communis stomate. right: Calcium Green-1 fluorescence in Commelina guard cells. Both photographs were made with a non-confocal microscope.
Stomatal pores control gas exchange in leaves. Changes in stomatal aperture are induced by many environmental factors, such as light and the phytohormone abscisic acid (ABA). The cytosolic Ca2+ concentration [Ca2+]cyt in guard cells is an important regulatory signal controlling stomatal aperture. Rises in cytosolic Ca2+ cause activation of guard cell anion efflux channels and downregulation of K+ uptake channels (Schroeder and Hagiwara, 1989), which provide mechanisms for Ca2+-dependent stomatal closing (Gilroy et al., 1990). The hormone ABA is known to induce increases in [Ca2+]cytin guard cells (McAinsh et al., 1990; Schroeder and Hagiwara, 1990; Gilroy et al., 1991), however, many of the molecular mechanisms in the signal transduction pathway, beginning with ABA receptors and leading to downstream targets remain unknown. In addition, several studies have shown that within populations of guard cells some cells do not show detectable increases in [Ca2+]cyt following exposure to ABA, leading to the suggestion that Ca2+ -independent ABA signaling pathways also exist in guard cells (Allan et al., 1994).
In previous studies, measurements of [Ca2+]cytin guard cells have been conducted using free acid forms of calcium sensitive fluorescent dyes (Grynkiewicz et al. 1985) introduced either by microinjection or by loading protoplasts during patch clamp experiments (Gilroy et al., 1990; McAinsh et al., 1990; Schroeder and Hagiwara, 1990). A limitation of these techniques is that only a single guard cell can be assayed at one time. In addition, these experimental approaches are more difficult using Arabidopsis due to the relatively small size of guard cells, though Arabidopsis mutants provide valuable backgrounds for studying ABA signaling in guard cells (Pei et al., 1997).
In plant cells, Ca2+-sensitive AM-ester dyes are usually degraded before loading by extracellular esterases or partitioned into plant vacuoles, hindering [Ca2+]cyt measurements. Here, we report on the development of a protocol for loading the calcium sensitive dye Calcium Green-1 AM into the cytoplasm of many guard cells within epidermal strips. The response of many guard cells to ABA could then be measured simultaneously. We show here unpublished results obtained with this method and also discuss limitations of the method. This procedure could be applied to loading of other ester probes into plant cells. We have subsequently adapted and developed other methods for loading of the acid forms of quantitative ratiometric calcium indicators fura-2 and Indo-1 into the cytoplasm of guard cells (K. Kuchitsu and Schroeder, data not shown). These methods for loading the ratiometric dyes Indo-1 and fura-2 and transgenic expression of cameleon Ca2+ indicator proteins are being published elsewhere (Allen et al., 1999; Allen et al., 1999b). We are depositing Calcium Green-1 AM-ester loading studies on the web, for those who are interested and because several laboratories have requested the protocol.
Plant Material
Young rosette leaves of Arabidopsis thaliana L. (Landsberg erecta ) were blended in cold distilled water for approximately 90 seconds (Pei et al., 1997). Epidermal fragments were collected on a polyethylene mesh (292 Ám, Spectrum). Isolated guard cells from Arabidopsis have previously been shown to be responsive to physiological stimuli (Pei et al., 1997).
Fully expanded leaves of Commelina communis were used. Lower (abaxial) epidermal strips were peeled manually from the leaves (Esser et al., 1997).
Calcium Green-1 loading
The general basis for acetoxymethyl (AM) ester loading of probes into cells is the following: the AM-ester form is membrane permeant and can enter cells. Hydrolysis by esterases within the cell releases the fluorescent probe which is not membrane permeant and is therefore trapped in the cytoplasm (Grynkiewicz et al. 1985).
In plant cells, it has been reported that extracellular esterases could severely reduce the loading of AM-esters into the cells. Therefore we applied eserine, an esterase inhibitor, to inhibit extracellular hydrolysis of the AM-ester. Eserine enhanced cytoplasmic dye loading.
Epidermes from Arabidopsis or Commelina were incubated for 20-30 minutes in the dark at 25░ C in loading solution: 100 mM KCl, 10 mM MES (pH 5.0 with KOH), 1 mM CaCl2, 300 ÁM eserine (physostigmine, Sigma) and 15 ÁM Calcium Green-1, AM (Molecular Probes). In some experiments dye loading was low and therefore dye concentrations and duration of the incubation were increased and epidermes were placed on a rotary shaker (0.5 Hz). After dye loading, the epidermal strips were washed with 100 mM KCl, 10 mM MES (pH 6.15 with KOH), 1 mM CaCl2.
Guard cells were imaged using two systems. One utilized an inverted fluorescence microscope with epifluorescence suitable for FITC. Quantitation of fluorescence changes was performed using a photomultiplier as described previously (Schroeder and Hagiwara, 1990) and recorded using Axotape software (Axon Instruments). For detailed spatial imaging studies with better z-axis resolution, confocal imaging studies were conducted using a Bio-Rad MRC-1000 laser scanning confocal system with an Ar-laser coupled to a Zeiss Axiovert 35M microscope.
Loading Commelina guard cells and measuring responses to ABA.
Figure 1 (left) shows a bright field image of a single stomatal complex of Commelina. Figure 1 (right) shows a fluorescence photograph illustrating the green fluorescence from the fluorescent dye Calcium Green-1 in the cytoplasm and nucleus of both guard cells from a different stomate. Guard cell vacuoles did not show bright fluorescence (Figure 1, right), contrary to the expected if the cleaved dye were loaded into vacuoles. This was further confirmed by confocal imaging studies. (Note that Figure 1 does not show confocal images). One advantage of using Calcium Green-1 vs. fluo-3 to develop loading protocols is that it is fluorescent at resting (sub-micromolar) calcium concentrations, allowing one to monitor loading (Fig. 1). Initial experiments to determine ABA effects on Commelina guard cells were performed using a conventional fluorescence microscope coupled to a photomultiplier. A pinhole diaphragm limited the area of detection to guard cells of a single stomatal complex, or to a single guard cell.
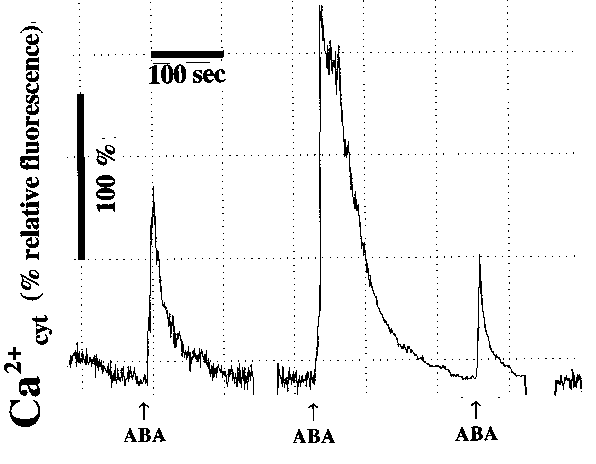
Figure 2: Abscisic acid causes transient [Ca2+] elevations in Commelina guard cells. (Cells were viewed between [Ca2+]cyt transients which caused interruptions in traces.)
Examples of recordings are presented in Figure 2. ABA was applied for short term exposures by local perfusion from a pipette to analyze effects of rapid exposure to ABA, as described previously (Schroeder and Hagiwara, 1990). Local application of ABA (100 ÁM) caused a transient increase in fluorescence indicating that cytosolic calcium increased. The fluorescence returned to background within 2-5 minutes. On the right side of Figure 2, an additional application of 100 ÁM ABA caused another increase in [Ca2+]cyt in the same guard cell. Under the imposed conditions over 50% of guard cells showed no [Ca2+]cyt elevation in response to ABA, as previously reported using other approaches (Allan et al., 1994; Gilroy et al., 1991; Schroeder and Hagiwara, 1990).
Using this system, two lines of experimentation were pursued to gain insight into the ABA-stimulated source(s) of calcium flux into the cytoplasm. Possible sources of calcium are the extracellular space and/or intracellular calcium stores such as the vacuole and the ER.
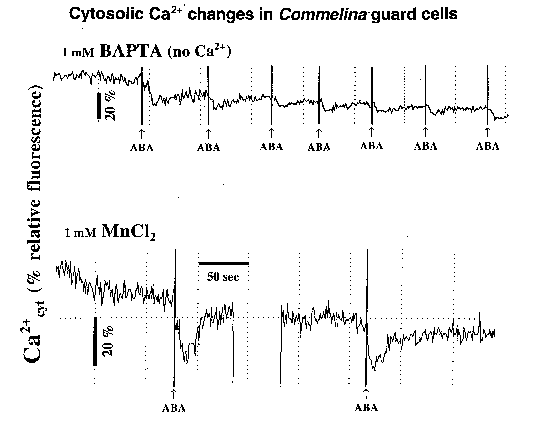
Figure 3: top: Extracellular BAPTA inhibits ABA-induced [Ca2+]cyt elevations. bottom: Extracellular manganese leads to quenching of Calcium Green-1 fluorescence upon local ABA application.
When calcium was omitted from the external solution and the calcium chelator BAPTA (1 mM) was included in the bath, ABA did not induce an increase in fluorescence (Figure 3, top). Vertical lines in Figure 3 are markers for times when ABA was locally applied to stomata. Interestingly, the small dips in fluorescence observed in response to ABA (Figure 3, top) are similar to CO2-induced [Ca2+]cyt changes in guard cells that were observed when epidermal strips were bathed in zero Ca2+ and EGTA (Webb et al., 1996). This indicates that ABA triggers influx of calcium from the extracellular space (Webb et al, 1996) (but does not rule out a contribution from internal Ca2+ stores during long-term ABA applications). Consistent with this hypothesis, when MnCl2 was included in the extracellular medium, ABA triggered a transient fluorescence decrease. When manganese binds to Calcium Green-1, it causes a quenching of the fluorescence. These initial studies indicate that :
1) influx of calcium from the extracellular space contributes to ABA-induced increases in [Ca2+]cyt,
2) the calcium influx pathway is permeable to Mn2+,
3) the transient activation of this influx pathway does not require extracellular Ca2+.
The results suggest that ABA induces calcium influx transients from the extracellular space. These data correlate closely to ABA-activated transient non-selective Ca2+-permeable channels, recorded by simultaneous patch clamping and fura-2 ratiometric photometry in Vicia faba guard cells (Schroeder and Hagiwara, 1990). Furthermore, patch clamp studies on Commelina guard cells showed transient and repetitive activation of non-selective currents by ABA (n=3), similar to those characterized in Vicia guard cells (data not shown).
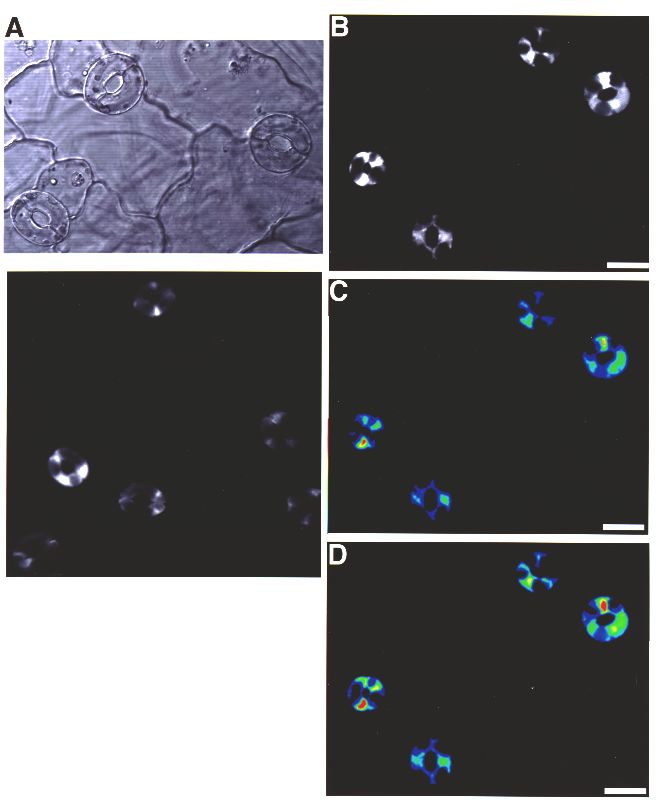
Because of the small size of Arabidopsis guard cells, confocal imaging was pursued. One advantage of this system is that fluorescence from several guard cells of an epidermal fragment can be imaged and measured simultaneously. In Figure 4, Calcium Green-1 loaded Arabidopsis guard cells are shown. The confocal transmission image (Figure 4A) shows a typical field of open Arabidopsis stomata.
 >
>
Figure 4: Loading of Calcium Green-1 into the cytoplasm of Arabidopsis guard cells. (A) Confocal transmission image of Arabidopsis stomates. (B) Confocal fluorescence image showing dye loading in 4 stomates. (C) Pseudo-colored image of (B) before ABA application. (D) Pseudo-colored image of (B) 18 seconds after ABA application. Image on left shows another example of Calcium Green-1 loaded guard cells. Some cells are not in the focal plane.
In Figure 4B, a confocal fluorescence image is shown of guard cells loaded with Calcium Green-1. As with Commelina, the confocal images clearly show that the cytoplasm of the guard cells is fluorescent and the vacuoles are dark, demonstrating that Calcium Green-1 is not loaded into vacuoles. Note that the nucleoplasm is also loaded with Ca2+-sensitive dyes, based on our confocal images, as is expected from experiments using AM-ester dyes in animal systems (Grynkiewicz et al. 1985). Furthermore, chloroplasts were loaded with dye, which we later circumvented by adapting a different method for loading the acid forms of the ratiometric dyes fura-2 and Indo-1 (K. Kuchitsu and J. Schroeder, unpublished). In experiments on cultured soybean culture cells using the AM-ester method described here, Calcium Green-1 was loaded into vacuoles showing that different cell types show different loading patterns (data not shown). False-color images are shown for the same guard cells before ABA application (Figure 4C) and 18 seconds after ABA application (Figure 4D). High resolution confocal analyses showed that fluorescence in the cytoplasm of guard cells increased with ABA application. Note that the intensity of the fluorescence is not identical in all cells in figure 4D, but all 8 guard cells showed an increase in fluorescence. The scale bar on the right of Figure 4 shows that higher intensities have green to red coloring (see: Limitations regarding qualitative nature of measurements). In some experiments no clear ABA-induced [Ca2+]cyt rises were resolved. The time course of the fluorescence changes of individual cells shown in Figure 4B to D can be seen in Figure 5A. ABA triggered a rise in [Ca2+]cyt commenced. The time course of calcium increases in response to rapid ABA application was similar to Commelina guard cells (Figures 2 and 5). Detailed studies showed that the decline in fluorescence in Arabidopsis guard cells was in part due to [Ca2+]cyt declining but also due to bleaching of the dye by the laser (Figure 5B) (see: Limitations). Note that in Figure 5 pre-ABA fluorescence intensities of single guard cells were all normalized (to 100%), showing similar relative increases. In control experiments where no ABA was added (ethanol solvent control), no increases in [Ca2+]cyt were observed (Figure 5B).
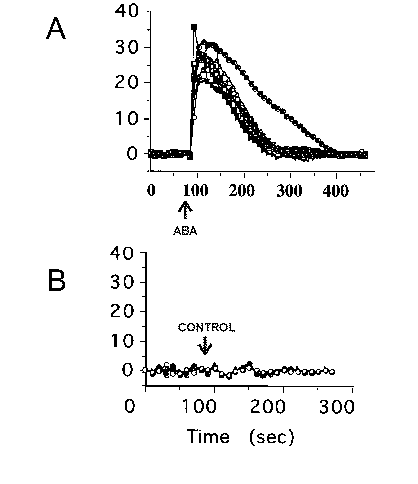
Figure 5>: (A) ABA-induced fluorescence increases were recorded in individual guard cells shown in figure 4B using image analysis software. Additional guard cells to those shown in figure 4B were analyzed in the same epidermis. (B) Control experiments in which the ethanol solvent was applied (at 90 sec) showed no increases in fluorescence.
Limitations:
Calcium Green-1 is a non-ratiometric Ca2+ dye and therefore only qualitative changes in [Ca2+]cyt could be observed. Furthermore, by pursuing many (>50 experiments) in Arabidopsis epidermes we found that it is critical that the epidermal strips do not move at all during confocal imaging because movements can cause apparent changes in fluorescence when using a single wavelength dye and laser confocal microscopy. We were able to circumvent these problems using software and hardware that rapidly alternates (every 0.5 sec) between collection of bright field confocal transmission images and fluorescent confocal images and by discarding any data in which movement of cells occurred. Movement of epidermes can be unequivocally determined by subtraction of successive bright field confocal images.
As mentioned in the Introduction, we subsequently circumvented these limitations by adapting and developing new methods for loading acid forms of ratiometric Ca2+ dyes into Arabidopsis guard cells (K. Kuchitsu and J. Schroeder, unpublished). Furthermore, methods were developed for fixing Arabidopsis epidermal strips, using a novel method that is very effective at avoiding movements, and for adapting non-confocal ratiometric imaging and photometry techniques to Arabidopsis guard cells (Allen, et al., 1999).
The new method also shows significantly reduced loading of dyes in the nucleoplasm and chloroplasts (Allen et al., 1999). In addition to loading of fura-2 and Indo-1 into Arabidopsis guard cells (Allen et al., 1999), we have more recently transgenically expressed the Ca2+-sensing cameleon protein in guard cells, which allows long term measurements of stimulus-induced cytoplasmic Ca2+ changes (Allen, et al., 1999b).
The present study provides a method for loading the AM-ester form of Calcium Green-1 specifically into guard cells. The procedure works well with both Commelina and Arabidopsis, and could generally be applied to loading of other ester probes into guard cells or into other plant cell types. Preliminary loading experiments on suspension cultured soybean cells showed vacuolar loading, suggesting that different cell types need to be tested for feasibility. The non-ratiometric nature of the dye does not allow quantitative analyses in Arabidopsis. Nevertheless, qualitative measurements could be pursued on the larger guard cells of Commelina communis. Commelina guard cells showed large fluorescence changes of >150%. For Arabidopsis studies epidermal movements could be ruled out by image analysis software. Data using this approach suggest that ABA induces transient calcium influx from the extracellular space in Commelina guard cells, similar to a previous study in Vicia guard cells (Schroeder and Hagiwara, 1990), and that this influx pathway is permeable to Mn2+. Furthermore, we have observed ABA-induced [Ca2+]cyt increases in Arabidopsis guard cells.
This research was supported by a National Science Foundation Grant (MCB9506191) (J.I.S.) and by an NIH grant to the UCSD National Center for Microscopy and Imaging Research (M.E.). We thank Dr. Maryann Martone for technical assistance with confocal microscopy.
Allan, A.C., Fricker, M.D., Ward, J.L., Beale, M.H., and Trewavas, A.J. (1994). Two transduction pathways mediate rapid effects of abscisic acid in Commelina guard cells. Plant Cell 6, 1319-1328.
Allen, G.J., Kuchitsu, K., Chu, S.P., Murata, Y. and Schroeder, J.I. (1999). Arabidopsis abi1-1 and abi2-1 phosphatase mutations reduce abscisic acid induced cytosolic calcium rises in guard cells. Plant Cell 11, 1785-1798
Allen, G.J., Kwak, J.M., Chu, S.P., Llopis, J., Tsien, R.Y., Harper, J.F., Schroeder, J.I. (1999b). Cameleon calcium indicator reports cytoplasmic calcium dynamics in Arabidopsis guard cells. Plant Journal (in press, October 1999).
Esser, J.E., Liao, Y-J. and Schroeder, J.I. (1997). Characterization of ion channel modulator effects on ABA- and malate-induced stomatal movements: Strong regulation by kinase and phosphatase inhibitors, and relative insensitivity to mastoparans. J. Exp. Bot. 48:539-550.
Gilroy, S., Read, N.D., and Trewavas, A.J. (1990). Elevation of cytoplasmic calcium by caged calcium or caged inositol trisphosphate initiates stomatal closure. Nature, 346, 769-771.
Gilroy, S., Fricker, M.D., Read, N.D. and Trewavas, A.J. (1991) Role of calcium in signal transduction of Commelina guard cells. The Plant Cell 3: 333-344.
Grynkiewicz G., Poenie, M., and Tsien, R.Y. (1985). A new generation of Ca2+ indicators with greatly improved fluorescence properties. J. Biol. Chem. 260:3440-3450.
McAinsh, M.R., Brownlee, C., and Hetherington, A.M. (1990). Abscisic acid-induced elevation of guard cell cytosolic Ca2+ precedes stomatal closure. Nature 343, 186-188.
Pei, Z-M., Kuchitsu, K., Ward, J.M., Schwarz, M. and Schroeder, J.I. (1997). Differential abscisic acid regulation of guard cell slow anion channels in Arabidopsis wild-type and abi1 and abi2 mutants. Plant Cell 9:409-423.
Schroeder, J.I. and Hagiwara, S. (1989). Cytosolic calcium regulates ion channels in the plasma membrane of Vicia faba guard cells. Nature 338:427-430.
Schroeder, J.I., and Hagiwara, S. (1990). Repetitive increases in cytosolic Ca2+ of guard cells by abscisic acid, activation of non-selective Ca2+ permeable channels. Proc. Natl. Acad. Sci. USA, 87, 9305-9309.
Webb, A.A.R., McAinsh, M.R., Mansfield, T.A. and Hetherington, A.M. (1996). Carbon dioxide induces increases in guard cell cytosolic free calcium. The Plant Journal 9: 297-304.