The Kiger Laboratory Research
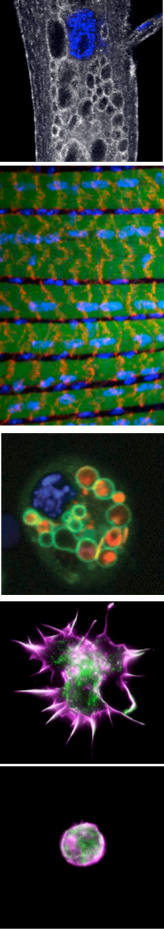
Cellular remodeling directed by phosphoinositide regulation. Phosphoinositide lipids, the seven different forms of phosphorylated phosphatidylinositol, provide a membrane “code” that controls a wide array of cellular functions. However, gaps remain in understanding the complex functional relationships between the relevant kinases, phosphatases and the specific roles mediated through phosphoinositide regulation. The large family of Myotubularin (MTM) phosphoinositide phosphatases (15 human, 7 fly, 1 yeast) are selective for PI(3)P and PI(3,5)P2 turnover, pointing to possible roles in complex regulation of endocytosis and/or autophagy. Further confounding the understanding of MTM family functions are that, curiously, half of the members encode for catalytically inactive, or “pseudo”-phosphatases. Importantly, mutations in MTM catalytic and pseudophosphatase members are both associated with human disease, including centronuclear myopathy (XLMTM) and Charcot-Marie-Tooth neuropathy (CMT4B). How potential MTM roles in endocytosis or autophagy relate to disease are not understood.
We identified mtm, a Drosophila MTM1/MTMR2 phosphoinositide phosphatase, as necessary and sufficient for formation of dynamic immune cell protrusions and immune cell recruitment to wounds. We determined that Mtm and Class II PI3-kinase, called Pi3K68D in flies, co-regulates a PI(3)P subpool on endosomes for endocytic recycling important for immune cell dynamic cortical functions. We have also demonstrated a central role for a Pi3K68D/Mtm pathway in phosphoinositide regulation of integrin endocytic trafficking crucial for maintenance of muscle attachments during muscle cell remodeling. We showed that mtm mutant myofibers exhibit hallmarks of the human XLMTM myopathy, and that the fly defects predicted disruption of integrin localization in human XLMTM. The identification of functionally distinct PI(3)P subpools under control of Class II PI3-kinase has broad implications, including possible strategies for treatment of MTM-related human disease.
In ongoing studies, we are addressing how phosphoinositide-directed endocytic recycling provides key input into the dynamic balance between distinct, cortical F-actin structures in immune cells. In muscle, we are exploring the regulation of integrin trafficking during cellular remodeling, as well as the relationships between mtm and orthologs of other genes also mutated in different forms of human centronucelar myopathy. We are pursuing how PI3KC2 and Mtm are spatiotemporally regulated. Important insights are coming from investigation of the MTM “pseudo”-phosphatase, Sbf, as a key coordinator of both PI3KC2/Mtm phosphoinositide regulation and membrane trafficking.
Regulation of cellular remodeling by autophagy.
Autophagy is the membrane-mediated process of cellular “self-eating” through encapsulation of proteins or organelles for lysosomal delivery and degradation, with well-known roles in stress responses. Autophagy has been the focus of growing interest due to its involvement in many processes, including immunity, neurodegeneration and cancer. We showed that autophagy is continuously required for macrophage cell spreading, with functions in proper cytoskeletal reorganization, and has a conserved role in the cortical remodeling of both Drosophila and mouse macrophages. Consequently, macrophages disrupted for autophagy are impaired in their recruitment to epidermal wounds. We showed a requirement for the p62 multiadaptor involved in selective targeting of ubiquitinated proteins for autophagic degradation, pointing to a role for selective autophagy (versus bulk autophagy) as a regulatory mechanism for cortical remodeling. This is the first demonstration of a true “cell remodeling” function – at the level of cell shape – for autophagy, which has been previously proposed but never shown.
In ongoing studies, we are continuing to address how autophagy specifically regulates immune cell remodeling, in part through the use of systems biology approaches to identify targets of selective autophagy. We are also investigating phosphoinositide regulation of autophagy.
Functional genomics of cellular remodeling.
The regulation of cell morphology can be viewed as a complex system that must integrate the temporal-spatial control of multiple cellular processes, including cytoskeletal and membrane dynamics, cell adhesion and growth. To understand morphogenetic programs, we are applying functional genomics to comprehensively probe the cellular-genetic networks that control cellular remodeling. We pioneered RNA-interference (RNAi) screens in Drosophila cells that permit unprecedented genome-wide loss-of-function analysis of metazoan cell morphology. Our RNAi microscopy screens have identified gene functions required to maintain distinct round or flat immune cell morphologies. Now, we are using RNAi screens to study how the addition of a hormonal cue culminates in the change of cells from a round to elongated cell shape, relevant to numerous cellular behaviors in vivo. Furthermore, co-RNAi screens targeting combinations of multiple genes are an ideal method for identifying new components of a pathway through suppressor or enhancer effects. In this way, we have isolated and continue to seek modifiers of specific phosphoinositide and autophagy pathways important in cellular remodeling.
Research Interests
Current Funding
National Institutes of Health NIGMS
The Hellman Family Foundation
San Diego Center
for Systems Biology
Past Funding
The David & Lucille Packard Foundation Fellowship in Science & Engineering
The March of Dimes
Basil O’Connor Award
The Sidney Kimmel Foundation for Cancer Research
Scholar Award